Abstract

Background A combination of cytarabine plus anthracycline according to the 7+3 schedule is the standard treatment backbone of fit newly diagnosed AML patients (pts). A daunorubicin dose of 90 mg/m2 is superior to 45 mg/m2, whereas little difference seems to be between 60 mg/m2 and 90 mg/m2. Double induction is commonly performed in younger pts in order to maximize dose intensity upfront. However, for pts with a good early response after first induction, there is no prospective randomized evidence on the necessity or value of a second induction cycle (IC).
Aims To provide randomized evidence for two fundamental questions in standard induction: First, is 60 mg/m2 really sufficient for induction or is 90 mg/m2 more efficacious? Second, can good responders after the first 7+3 induction be spared a second IC?
Methods DaunoDouble was a two-part, two-arm open-label multicenter prospective randomized phase III trial. Pts 18-65 years with newly diagnosed AML, normal cardiac and organ function received a first IC with seven days of cytarabine plus three days of daunorubicin ("7+3") with a 1:1 stratified randomization for 60 versus (vs) 90 mg/m2 (Dauno60 vs Dauno90). Response assessment in bone marrow was evaluated by cytology on day 15. A blast count <5% was defined as good response, which was the primary endpoint of this first randomization step.
Good responders were randomized to receive a second IC (arm D) or no second IC (arm S). In arm D, the second IC contained 60 mg/m2 after Dauno60 and 45 mg/m2 after Dauno90. Primary endpoint of the second randomization was CR/CRi after completion of induction. We assumed non-inferiority of single induction in terms of CR/CRi rate, based on a margin of 7.5%.
Results Between 2014 and 2022, 864 pts were enrolled and received the first IC with 7+3. Median age was 52 years, 88% had de novo AML. Favorable, intermediate and adverse risk (ELN 2017) was present in 37%, 46% and 17% of pts, respectively. No significant imbalances were observed between the two treatment arms except for a higher NPM1 mutant rate in the Dauno90 arm (32.4% vs 41.7%, p=0.034).
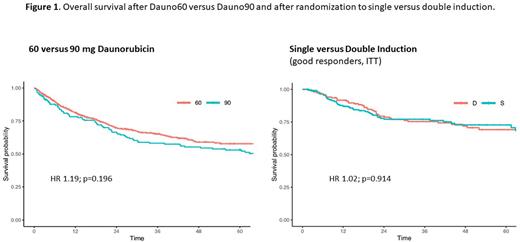
A pre-planned interim analysis after the first randomization of 218 pts revealed a statistically and clinically non-significant difference of 42% vs 49% good responders after first induction with 60 vs 90 mg/m2 daunorubicin (p=0.341). Based on this result, the first randomization step was suspended and all consecutive pts received 60 mg/m2 in induction I. At the end of enrollment, 707 and 157 pts have received 60 or 90 mg/m2, with a corresponding proportion of good early responders of 44.4% vs 47.8% (p=0.930) and a CR rate of 89.6% vs 88.5% after the end of induction (p=0.691). After a median follow-up of 43.6 months, 3-y RFS after Dauno60 vs Dauno90 was 53.8% vs 50.1% (p=0.561) and 3-y OS 65.2% vs 58.3% (p=0.196). During first induction, 57% of pts in Dauno60 and 58% in Dauno90 experienced an AE ≥ grade 3 (p=0.877); mortality in induction I was 2.3% and 4.7%, respectively (p=0.109).
After IC I, a marrow blast clearance below 5% on day 15 was achieved in 389 pts (45%), providing eligibility for the second randomization. Of these pts, 189 were randomized into arm S and 187 into arm D (ITT population). CR/CRi rates at the end of induction were 85.2% after single induction and 85.6% after double induction, resulting in a CR difference of 0.4% (p for non-inferiority test 0.0269). The CR/CRi rates in 326 pre-defined per-protocol pts (PP) were 86.8% vs 91.5%, resulting in a CR difference of 4.7% (p=0.205). Until the end of induction, 58% of pts in the S arm and 65% of pts in the D arm experienced an AE ≥ grade 3 (p=0.195). Early mortality 60 days after induction start was 0.6% in both arms.
After a median follow-up time of 43.6 months, 3-y RFS after single vs double induction was 51% vs 60%, which was non-significant in the ITT population (HR 1.35; p=0.074) and borderline significant in the PP population (HR 1.43; 0=0.05), but not significant in multivariable analyses. OS after 3 years was identical with 77% vs 75% after single or double induction in the ITT (HR 1.02; p= 0.914) and 77% vs 76% in the PP population (HR 1.12; p=0.628).
Conclusion The use of 90 mg daunorubicin in the context of classical 7+3 induction did not lead to higher remission rates or longer survival than 60 mg. In pts with a good early response after first induction, a second induction had only limited impact on RFS. Double induction did not lead to an overall survival benefit in pts with a good early response first 7+3 induction.
Disclosures
Röllig:BMS: Consultancy, Honoraria; Amgen: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Servier: Consultancy; Jazz: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria. Steffen:Jazz Pharmaceuticals: Other: Travel/Congress Participation Support; AbbVie: Other: Travel/Congress Participation Support. Schliemann:Jazz: Consultancy, Research Funding; Boehringer-Ingelheim: Research Funding; Astrazeneca: Consultancy; Astellas: Consultancy; Abbvie: Consultancy, Other: travel grants; Philogen S.p.A.: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Other: travel grants; Novartis: Consultancy; Roche: Consultancy; Pfizer: Consultancy. Alakel:Pfizer: Consultancy, Honoraria. Haenel:Takeda: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pfizer: Honoraria; Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria; JAZZ: Consultancy, Honoraria. Sauer:Pfizer: Honoraria; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Ridgeline Discoveries: Membership on an entity's Board of Directors or advisory committees. Jost:Jazz: Honoraria; BMS Celgene: Honoraria. Krause:Art-tempi: Honoraria; Kosmas: Honoraria; Abbvie: Other: Expenses. Hochhaus:Incyte: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Bristol Myers Squibb: Research Funding. Schmid:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Research Funding; Abbvie: Research Funding. Fransecky:Pfizer: Consultancy, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau. Schetelig:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Middeke:Abbvie: Membership on an entity's Board of Directors or advisory committees. Thiede:Kronos Bio, Inc.: Honoraria; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen Pharmaceuticals: Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AgenDix GmbH: Current Employment, Current equity holder in private company.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal